9-Energy-chenges-and-reversible-reaction



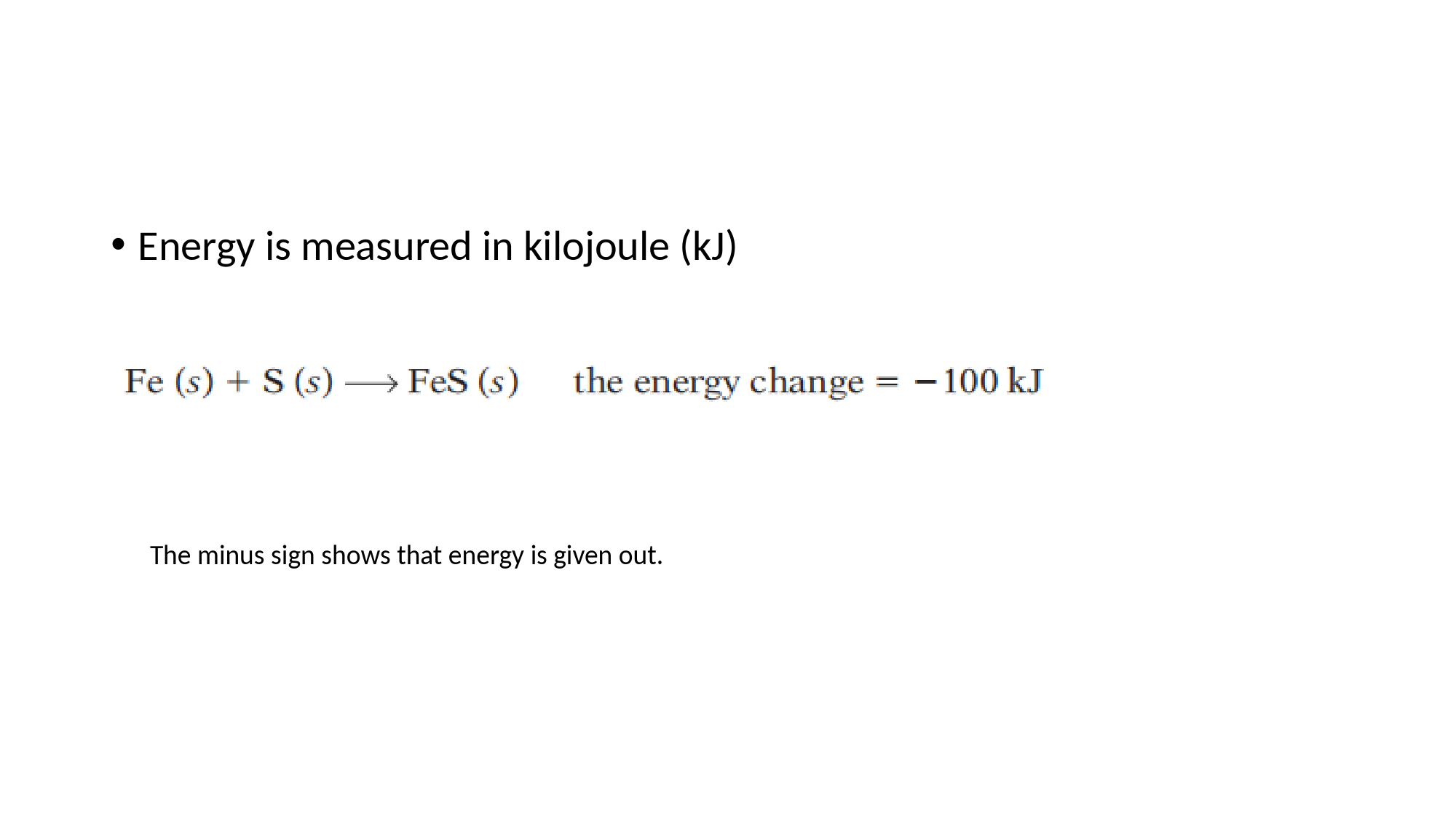
Chapter9Energychanges,andreversiblereactions9.1Energychangesinreactions•Energychangesinreactions•Duringachemicalreaction,thereisalwaysanenergychange.•Soreactionscanbedividedintotwogroups:exothermicandendothermic.Exothermicreactions•Exothermicreactionsgiveoutenergy.Sothereisatemperaturerise.•Thesereactionscanbedescribedas:energyleveldiagram•Energyismeasuredinkilojoule(kJ)Theminussignshowsthatenergyisgivenout.Otherexamplesofexothermicreactions•Alltheseareexothermic:•theneutralisationofacidsbyalkalis.•thecombustionoffuels.Weburnfuelstoobtainheatforcooking,heatinghomes,andsoon.Themoreenergytheygiveout,thebetter!•respirationinyourbodycells.Itprovidestheenergytokeepyourheartandlungsworking,andforwarmthandmovement.Endothermicreactions•Endothermicreactionstakeinenergyfromtheirsurroundings.•Thesereactionscanbedescribedas:energyleveldiagram•TheenergychangeTheplussignshowsthatenergyistakenin.Otherexamplesofendothermicreactions•reactionsthattakeplaceincooking•photosynthesis.Thisistheprocessinwhichplantsconvertcarbondioxideandwatertoglucose.Itdependsontheenergyfromsunlight.•1Isitexothermicorendothermic?•atheburningofacandle•bthereactionbetweensodiumandwater•cthechangefromraweggtofriedeggexothermic(butneedsenergytostartitoff)exothermicendothermic•2Whichunitisusedtomeasureenergychanges?•kilojoule(kJ)Theenergychangeforthisreactionis-822.4kJ.Whatcanyouconcludeaboutthereaction?Itisexothermic1aneutralisationbItgivesoutenergy.cItwillrise.dThediagramshouldbeliketheoneonpage110,butwithNaOH(aq)andHCl(aq)asreactants,andNaCl(aq)andH2O(l)asproducts.•Drawanenergyleveldiagramfor:•aanendothermicreactionbanexothermicreaction9.2Explainingenergychanges•Makingandbreakingbonds•Breakingbondstakesinenergy.Makingbondsreleasesenergy.Example1:anexothermicreaction•Hydrogenreactswithchlorineinsunshine,toformhydrogenchloride:Iftheenergytakenintobreakbondsislessthantheenergyreleasedinmakingbonds,thereactionisexothermic.Example2:anendothermicreactionIftheenergytakenintobreakbondsisgreaterthantheenergyreleasedinmakingbonds,thereactionisendothermic.Bondenergies•Theenergyneededtomakeorbreakbondsiscalledthebondenergy•Thebondenergyistheenergyneededtobreakbonds,orreleasedwhenthesebondsform.ItisgiveninkJ/mole.Calculatingtheenergychangesinreactions•1.Theexothermicreactionbetweenhydrogenandchlorine•2TheendothermicdecompositionofammoniaStartingareactionofi•1Twostepsmusttakeplace,togofromreactantstoproducts.•Whatarethey?•1Bondsmustbebroken,thennewbondsmustform.•2.Somereactionsareendothermic.Explainwhy,usingthe•ideasofbondbreakingandbondmaking•2Theenergythathastobeputintobreakbondsisgreaterthanthe•energygivenoutwhennewbondsform.•3Hydrogenreactswithoxygen.Drawtheequationfor•thereactionasabove,withlinestoshowthebonds.•Nowseeifyoucancalculatetheenergychangeforthe•reactionin3,usingthebondenergytableonpage112.9.3Energyfromfuels•Whatisafuel?•Afuelisanysubstanceweusetoprovideenergy.•Thefossilfuels•Thefossilfuels--coal,petroleum(oil),andnaturalgas(methane)--arethemainfuelsusedaroundtheworldTheworldusesupenormousquantitiesofthefossilfuels.Forexample,nearly12milliontonnesofpetroleumeveryday!Sowhatmakesagoodfuel?•Thesearethemainquestionstoaskaboutafuel:•Howmuchheatdoesitgiveout?•Doesitcausepollution?•Isiteasilyavailable?•Isiteasyandsafetostoreandtransport?•Howmuchdoesitcost?Twofuelsgrowinginimportance•Becauseoffearsaboutglobalwarming,thereisapushtousenewfuels.Likethesetwo:•EthanolThisisanalcohol,withtheformulaC2H5OH•HydrogenThisgasburnsexplosivelyinoxygen,givingoutalotof•energy--soitisusedtofuelspacerockets.ItisalsousedinfuelcellsTwofuelsgrowinginimportance•Becauseoffearsaboutglobalwarming,thereisapushtousenewfuels.Likethesetwo:•EthanolThisisanalcohol,withtheformulaC2H5OH•HydrogenThisgasburnsexplosivelyinoxygen,givingoutalotof•energy--soitisusedtofuelspacerockets.ItisalsousedinfuelcellsDifferentamountsofheatNuclearfuels•Nuclearfuelsarenotburned.TheycontainunstableatomscalledradioisotopesNuclearfuelhastwobigadvantages:•Itgivesouthugeamountsofenergy.Apelletofnuclearfuelthesizeofapeacangiveasmuchenergyasatonneofcoal.•Nocarbondioxideorotherpollutinggasesareformed.•Q:•aSketchanenergyleveldiagramthatyouthinkshows:•iagoodfuel•iiaverypoorfuel•Thefuelbutane(C4H10)burnstogivethesameproductsas•methane.Writeabalancedequationforitscombustion.9.4Givingoutenergyaselectricity•Electricity:aformofenergy•Electricityisacurrentofelectrons.Likeheat,itisaformofenergy.•Electricityfromaredoxreaction•Sowhatisgoingon?•1.•2.Theelectronsflowalongthewiretothecopperstrip,asacurrent.•3.•Soaredoxreactionisgivingoutenergyintheformofacurrent.Asimplecell•Themetalstrips,wire,andbeakerofsolutionaboveformasimplecell.•Electronsflowfromthemagnesiumstrip,soitiscalledthenegativepole.•Thecopperstripisthepositivepole.Thesolutionistheelectrolyte.•Asimplecellconsistsoftwometalsandanelectrolyte.Themorereactivemetalisthenegativepoleofthecell.Electronsflowfromit.•Inasimplecell,whichmetalgivesupelectronstoproducethecurrent:themorereactiveorlessreactiveone?2themorereactiveoneInasimplecell,whichmetalgivesupelectronstoproducethecurrent:themorereactiveorlessreactiveone?No,thetwostripsmustbeofdifferentmetals.Themorereactivemetalwillgiveupelectrons,toformacurrent.•aSketchanenergyleveldiagramthatyouthinkshows:•iagoodfueliiaverypoorfuel•bWhatelsedoyouneedtothinkabout,todecide•whetherasubstancewouldmakeagoodfuel?•1aiAgoodfuelgivesoutplentyofenergy.Soonthe•energyleveldiagramtheproductsshouldbeatamuchlowerenergy•levelthanthereactants.•iiAverypoorfuelwillgiveoutverylittle•energy,sotheenergygapshouldbeverysmall.•bThinkabout:•pollution,reliabilityofsupply,easeandsafetyofstorageanduse,•cost•Lookatthetableabove.Fromalltheinformationgiven,•whichofthethreefuelsdoyouthinkisbest?Explain.•2Hydrogengivesoutmostheatpergram,andproducesonly•water.Thismakesitanattractivefuel.(Butitishighlyflammable,•sosafetyisanissue.)•4aTheenergygivenoutwhennewbonds•formisgreaterthantheenergyneededtobreakbonds.•iiSomeenergymustbeputin,tostartthebondsbreaking.Aspark•orflamecanprovidethisenergy.ciEnergyisgivenout,overall.•iiexothermicd55.6kJThehydrogenfuelcellAdvantagesofthehydrogenfuelcellAdvantagesofthehydrogenfuelcell•Onlywaterisformed.Nopollutants!•Thereactiongivesoutplentyofenergy.•Wewillnotrunoutofhydrogen.Itcanbemadebytheelectrolysisofwaterwithalittleacidadded.Butthereisadrawback.Hydrogenisveryflammable.Asparkorlitmatchwillcauseamixtureofhydrogenandairtoexplode.Soitmustbestoredsafely.•aInthehydrogenfuelcell,whatisthefuel?•bHowaretheelectronstransferredinthiscell?9.5Reversiblereactions•Whenyouheatcopper(II)sulfatecrystals…9.5Reversiblereactions•Whenyouheatcopper(II)sulfatecrystals…Sothereactioncangoineitherdirection:itisreversible.•Thereactionwestartwith(1above)iscalledtheforwardreaction.Reaction2istheback(reverse)reaction.Weusethesymbolinsteadofasinglearrow,toshowthatareactionisreversible.Sotheequationforthereactionaboveis:Whatabouttheenergychange?•Areversiblereactionisendothermicinonedirection,andexothermicintheother.Thesameamountofenergyistransferredeachtime.SomeimportantreversiblereactionsReversiblereactionsandequilibriumEquilibriummeansthereisnooverallchange.dynamicmeansthereiscontinualchange:ammoniamoleculescontinuallybreakdown,whilenewonesform.•Inaclosedsystem,areversiblereactionreachesastateofdynamicequilibrium,wheretheforwardandbackreactionstakeplaceatthesamerate.Sothereisnooverallchange.ThetermdynamicequilibriumisusuallyshortenedtoequilibriumAchallengeforindustry•1.Whatisareversiblereaction?•2.Explainthetermdynamicequilibrium•3.Nitrogenandhydrogenaremixed,tomakeammonia.•aSoon,tworeactionsaregoingoninthemixture.Givetheequationsforthem.•bForatime,therateoftheforwardreactionisgreaterthantherateofthebackreaction.Hasequilibriumbeenreached?Explain.9.6Shiftingtheequilibrium•ThechallengeWhatcanbedone?•Youwantasmuchammoniaaspossible.Sohowcanyouincreasetheyield?•Thisidea,calledLeChatelier’sprinciple,willhelpyou:•Whenareversiblereactionisinequilibriumandyoumakeachange,thesystemactstoopposethechange,andrestoreequilibrium.Anewequilibriummixtureforms.1ChangethetemperatureWillraisingthetemperaturehelpyouobtainmoreammonia?•Increasingtemperaturemakesthereactionmoveinthedirectionthattakein(endothemicreaction)Whatifyoulowerthetemperature?•lowertemperaturemakesthereactionmoveinthedirectionthatgiveout(exothemicreaction)2ChangethepressureIfthenumberofmolesofgasisthesameonbothsidesoftheequation,changesinvolumeandpressurehavenoeffectontheequilibrium.3Removetheammonia•Moreammoniawillform,torestorethebalance.4Addacatalyst•Acatalystspeedsuptheforwardandbackreactionsequally.•Sothereactionreachesequilibriumfaster,whichsavesyoutime.•Buttheamountofammoniadoesnotchange.•Choosingtheoptimumconditions•Sotogetthebestyieldofammonia,itisbestto:•usehighpressure,andremoveammonia,toimprovetheyield•useamoderatetemperature,andacatalyst,togetadecentrate.•Page223showshowtheseideasareappliedinanammoniafactory.•Anoteaboutrate•Bynow,youshouldrealisethat:•achangeintemperaturealwaysshiftsequilibrium:itchangestheyield.•achangeinpressurewillshiftequilibriumonlyifthenumberof•moleculesisdifferentoneachsideoftheequation.•Sulfurdioxide(SO2)andoxygenreactexothermicallytoformsulfurtrioxide(SO3).Thereactionisreversible.•aWritethesymbolequationforthisreaction.•bWhathappenstotheyieldofsulfurtrioxideifyou:•iincreasethepressure?iiraisethetemperature?
提供9-Energy-chenges-and-reversible-reaction会员下载,编号:1701027525,格式为 xlsx,文件大小为75页,请使用软件:wps,office Excel 进行编辑,PPT模板中文字,图片,动画效果均可修改,PPT模板下载后图片无水印,更多精品PPT素材下载尽在某某PPT网。所有作品均是用户自行上传分享并拥有版权或使用权,仅供网友学习交流,未经上传用户书面授权,请勿作他用。若您的权利被侵害,请联系963098962@qq.com进行删除处理。

 下载
下载 下载
下载 下载
下载 下载
下载 下载
下载 下载
下载 下载
下载 下载
下载 下载
下载 下载
下载 下载
下载 下载
下载